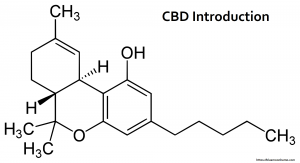
Blue Moon Hemp – DEA Ruling is Good News
By Christopher D. Cowart
December 15, 2016
Fort Lauderdale, FL – The announcement in the Federal Register yesterday by the DEA addressing the change in assigning a code to extracts from Cannabis is welcome news. Although some have misinterpreted this as a negative development, it is instead a necessary and valid next step in the development and commercialization of Cannabis extracts by the buying public. This is a classification issue and not an enforcement issue.
There are several direct benefits from the new coding:
- As stated, the primary driver is the import / export of these extracts. Previous to this, all derivative products were grouped under “marijuana’, which leads to confusion at border crossings and customs offices both here and abroad. This will clearly designate those extracts which have <.3% THC as a derivative and subject to laws and import / export restrictions accordingly. This should make it simpler to buy / sell extracts to / from other countries.
- The government, contrary to popular belief, does do some forward thinking occasionally. We interpret this as measure as a way to divide these extracts from the group of “marijuana” with the prospect of achieving two major objectives.
- Provide a mechanism to tax and collect revenue on a new subset of the MMJ space.
- Provide a means to eventually separate Cannabis Sativa (Industrial Hemp) from the Cannabis Indica (Marijuana) and move it into an Agricultural Product where it rightly belongs. Mitch McConnell is on record supporting this and there is legislation moving forward designed to accomplish this re-classification.
- Because much of the work being done domestically is under research and development license, with grants provided by the U.S.D.A. and State agencies as well, this new classification will allow those funding mechanisms to be clear in whether they are funding Low T (THC) or High (T) programs. This is important in the continuing development of these research programs which are closely linked to the NIH (National Institute of Health) and various other research entities.
In summary, we view this as a necessary next step which has been taken by the Government. It tacitly serves as an acknowledgement that this is a growing industry. They are taking measures to do what Government does, regulate, tax and monitor the commerce and development of this industry. The Schedule I classification remains the same, as does the unanimous 2004 9th Circuit Ruling click here which allows for the commercial use of Hemp derivatives if the THC level is <.3 %. Blue Moon Hemp, Inc. products are all THC = ZERO, so we have NO compliance issues and all of our source material is obtained with the necessary compliance and licensure required. There is a reason this segment is growing – the products are effective and provide a benefit to the end use. Please visit www.bluemoonhemp.com for more information and to view our selections.
Contact
To learn more about Blue Moon Hemp, Inc.
Or Wholesale Opportunities
Christopher D. Cowart
President / CEO
P.O. Box 461060
Ft. Lauderdale, FL 33346
Office: (844) 425-8666
Direct: (954) 802-8826
chris@wholesale.swissrelief.com
These comments and views are the opinion of Blue Moon Hemp, Inc. and are not meant to infer or convey any legal opinion or doctrine. All parties who view this must rely on their own research and data to draw their own conclusions.